Reflux heating
Reflux heating
Learning objectives
- To illustrate a reflux heating setup.
Temperature is one of the kinetic factors most often used to modify the rate of a chemical reaction.
Cooler temperatures will slow or stop a reaction. It is the function of the refrigerator that prevents rapid transformation (degradation) of food.
Conversely, a higher temperature can often accelerate the reaction rate. But we should take precautions. Prolonged heating may cause the complete evaporation of the solvent. If the ball is blocked, the gases would dangerously increase the pressure.
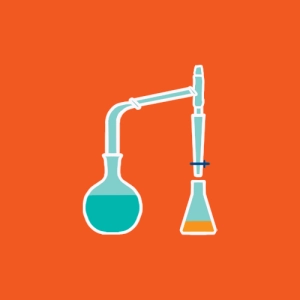
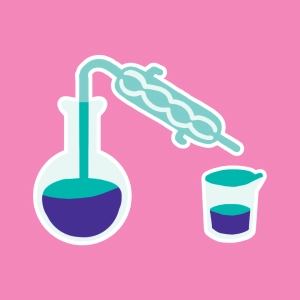
The animation shows a typical heater that can reflux heat nbsp;a reaction mixture at constant pressure (atmospheric pressure), without loss of material (produced vapors are condensed and returned to the mixture).

Discover EduMedia for free
The interactive encyclopedia that brings science and math to life in the classroom.
Over 1,000 resources