 Radioactive decay #1
Radioactive decay #1
Learning objectives
- To distinguish the different types of radioactive decay.
- To specify that nearly all alpha decay or beta decay are accompanied by gamma emissions.
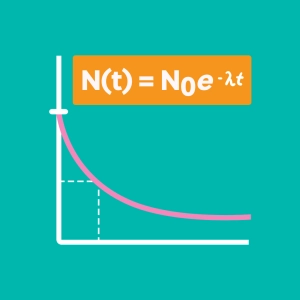
- To define the becquerel (Bq), a unit of measurement of activity in a radioactive sample (number of decays per second).
- To show that there are forms of ionizing radiation with very different penetrating properties. Gamma photons have strong penetrating properties which are used in nuclear medicine.
Contrary to popular opinion, radiation is a natural phenomenon. It was discovered in 1896 by A.H. Becquerel who was studying the fluorescent properties of uranium salts.
We identify 3 distinct types of radioactive decay. Each type of decay liberates a large amount of energy:
- Alpha radiation: the emission of a helium nucleus.
- Beta (+ and -) radiation: the emission of either an electron (beta-) or a positron (beta+).
- Gamma emission: the liberation of a high-energy photon.

Discover EduMedia for free
The interactive encyclopedia that brings science and math to life in the classroom.
Over 1,000 resources