 Electronic configuration
Electronic configuration
Learning objectives
- To illustrate the quantization of energy levels
- To define the concepts of spin and orbitals
- To make the link between the conventional writing of the electronic configuration, and the representation of the atom (Bohr model).
- To know how to find one's way in Mendeleev's table based on the electronic configuration.
- To write the electronic configuration of any element of the periodic table.
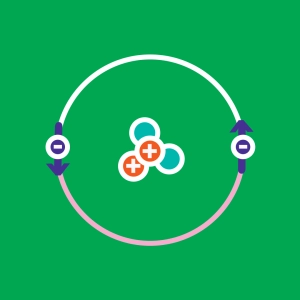
The electrons of an atom are distributed over very specific atomic orbitals. The electronic configuration describes this electronic distribution around the nucleus. Orbitals are complex shapes that are determined using quantum mechanics. The same atom can have several electronic configurations, and therefore, several energy states. The lowest energy configuration is called the ground state. All other configurations correspond to "excited states".
The logic of this animation follows Hund's rule. Note that some elements such as gold are exceptions to this rule.
Abbreviated writing of the electronic configuration: writing the electronic configuration of an atom quickly becomes tedious. Let's take the example of the zinc atom (Zn). Its configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 . It is customary to abbreviate this notation using the noble gas that precedes zinc in the periodic table, namely Argon (Ar). Thus, the electronic configuration of Zn can be more compactly written [Ar] 4s2 3d10, which corresponds to the configuration of argon supplemented by 2 electrons in the s orbital of level 4 (4s2) then 10 electrons in the d orbitals of level 3 (3d10).
Thanks to the IAEA (International Atomic Energy Agency) for its valuable source of information that is The IAEA's NUCLEUS information resource portal. Its API (Livechart Data Download API) was of great help in developing this educational animation.

Discover EduMedia for free
The interactive encyclopedia that brings science and math to life in the classroom.
Over 1,000 resources