 Atom builder
Atom builder
Learning objectives
- To represent each element of Mendeleev's periodic table.
- To define the atomic number and mass number.
- To distinguish the names and properties of the different elementary particles (proton, neutron, electron).
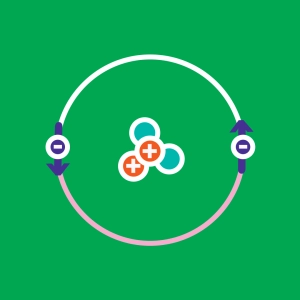
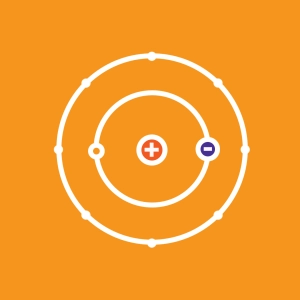
All matter is made up of atoms, and all atoms are made up of three main particles: protons, neutrons, and electrons. Protons and neutrons stick together to form the nucleus while electrons orbit around it.
These three main particles have both a mass and a charge:
- Mass of a proton equals the mass of a neutron: 1.67 x 10-27 kg. But the mass of an eletron is 2000 times less the mass of a proton. This explains why we affirm that all of the mass of an atom comes from the nucleus, with a very small contribution from the electron cloud.
- The electric charge of a proton is 1.6 x 10-19 C. As its name implies, neutrons are neutral (charge = 0). As a result, the nucleus of any element is positively charged. The electric charge of an electron is the exact opposite of the one of the proton: -1,6 x 10-19 C. The vast majority of the elements we find in the nature are neutral because the number of electrons balance precisely the number of protons. If not, we say such an atom is ionized.
The [kg] and [C]oulomb I.S units for mass and charge are not adapted for such tiny particle so we usually prefer to express the mass and charge with reduced unit:
- Proton: Mass = 1 Charge = +1
- Neutron: Mass = 1 Charge = 0
- Electron: Mass ≈ 0 Charge = -1
Thank you to The IAEA's NUCLEUS information resource portal, the Livechart Data Download API was a great help to develop this highly valuable teaching material.
Simulator Controls: Generate an atom using the "particle" buttons. Click the arrow to open the periodic table of Mendeleev. Click the eye to erase or reveal table elements. Each box, line and column of the table is clickable, as are the buttons for mass number, atomic number and charge.

Discover EduMedia for free
The interactive encyclopedia that brings science and math to life in the classroom.
Over 1,000 resources